Which of the Following Substances Has the Highest Melting Point
All the elements in the group form basic oxides 3. We can say that the symmetry of the compound goes in the same manner as melting point.

Which Compound Has Highest Melting Point Youtube
Based on Coulombs law of interaction strength between ions which of the following compounds has the highest melting point.

. The electronegativity of Be Hg Ca and Cl respectively are 1572010 and 316. Therefore it fits more closely in the crystal lattice and hence more energy is required to break the crystal lattice of p-dichlorobenzene. 3 rows Delta_UHTheta lattice energy prop Charge on the ions prop Comparable size of ions Therefore.
O - dichlorobenzene m - dichlorobenzene and p - dichlorobenzene. F2 Cl2 Br2 I2 Which substance has the highestlowest meltingboiling point etc. Boron trifluoride bf3 3.
My melting point range was 105-115. BeCl2 HgCl2 and CaCl2 are the examples of Chloride compounds. The melting point is directly proportional to the symmetry of the compound.
Rank the following liquids in order of increasing boiling points from lowest to highest. MgO Magnesium oxide is an ionic solid in which there are strong force. The melting point of pure acetylsalicylic acid is 135 degrees celsius.
M g O has a high melting point because of the difference in lattice energy. Because magnesium oxide is composed of ions with two charges Mg 2 and O2- it has a substantially higher melting temperature of 2852 degrees Celsius. Melting points depend upon lattice energy N aCl and K Cl have unit charge on their ions while M gO and BaO have two units of charge therefore lattice energies of M gO and BaO are expected to be larger than those of N aCl and K Cl.
Which of the following ionic compounds has the highest melting point. 100 3 ratings 1 Answer. Which of the following should have the largest.
View the full answer. Salt has the highest melting point among the choices because of its strong ionic bonds thereby more energy is required to break the bonds therefore melting them. Which has the higher melting point.
None of these have dipoles. The density of the elements increases down the group 4. The elements in a group of the Periodic Table show the following trends.
Which of the following substances will have the highest melting point. B dissolve in water. Note that ionic compounds have high melting points because the electrostatic forces holding the ions ion-ion interaction are much stronger.
Among the present substances salt has a melting point of about 801 degrees Celsius. Lattice energy is inversely proportional to the size of ion. Which one of the following substances would be expected to have the highest melting point.
Cs 2 O. Since M g2 is smaller than Ba2 therefore M gO has the higher lattice energy and hence has the higher melting point. C have melting points lower than those of molecular compounds.
Of all the substances present a sodium chloride or salt has the highest melting temperature. He NH 3 Ar N 2. Since M g2 is smaller than Ba2 and having 2 charge M gO will have the highest melting point from the given compounds.
Therefore magnesium oxide has the higher melting point. Tungsten is the chemical element with the highest melting point at 3414oC. Melting point depends upon lattice energy.
Tantalum Hafnium Carbide Alloy 3990 Tantalum hafnium carbide alloy takes the 1st place in our list of the materials with the highest melting point. Oxide anion is a better candidate as compared to the chloride anion. So p-dichlorobenzene is more symmetrical than the o- and m- isomers.
NaCl and KCl have unit charge on their ions whereas MgO and BaO have two units of charge. The melting point of the elements decreases down the group In which group are the elements. A form solutions that conduct electricity well.
I do not even know where to start for this. So I2 has the strongest forces and F2 will have the weakest. Which of the following compounds will have the highest melting point and why.
Comment on the purity of your product based on its melting point range. Hence lattice energies of MgO and BaO are expected to be larger than those of NaCl and KCl. We know greater the electronegativity difference greater will be the ionic character of the bonds between elements in molecules and as a consequence greater will be the melting point of the compounds.
This means that the compound will exhibit the highest lattice energy as well. C have melting points lower than those of molecular compounds. This property makes tungsten use as electrical filaments in incandescent lamps.
Tantalum hafnium carbide alloy Ta4HfC5 actually refers to tantalum and hafnium pentacarbonate compound which has the highest melting point among known compounds. Because NaCl has the strongest intermolecular forces it will have the highest melting point. Correspondingly I2 will have the highest boiling point and F2 will have the lowest boiling point.
A CaCl 2 b NaCl c NaF d MgO. Of these options NaCl is the only ionic solid whereas the other options are held together by dipole-dipole forces NO₂ and H₂S or dispersion forces Cl₂ and CH₄. Bigger molecules will have stronger London dispersion forces.
A He N 2 Ar NH 3 b He Ar N 2 NH 3 c NH 3 Ar N 2 He d NH 3 N 2 Ar He. View more similar questions or ask a new question. Highest melting point will be for E MgO Explanation- Ionic solids have very high melting point because of strong electrostatic forces of attraction between them.
Sodium chloride has a melting point of 801 C and is composed of Na ions and Cl-ions. They all have the same FCC lattice structure but the lattice energy of M g O is roughly 4 times that N a C l and K C l because it is proportional to ZZ-. D require more energy to change from the liquid to the gaseous state.
The element with the lowest proton number has the lowest reactivity 2. Furthermore M g 2 and O 2 both have an electron configuration of N e while B a 2 has a X e s one. Since Mg 2 is smaller than Ba 2 hence MgO has the higher lattice energy and thus has.
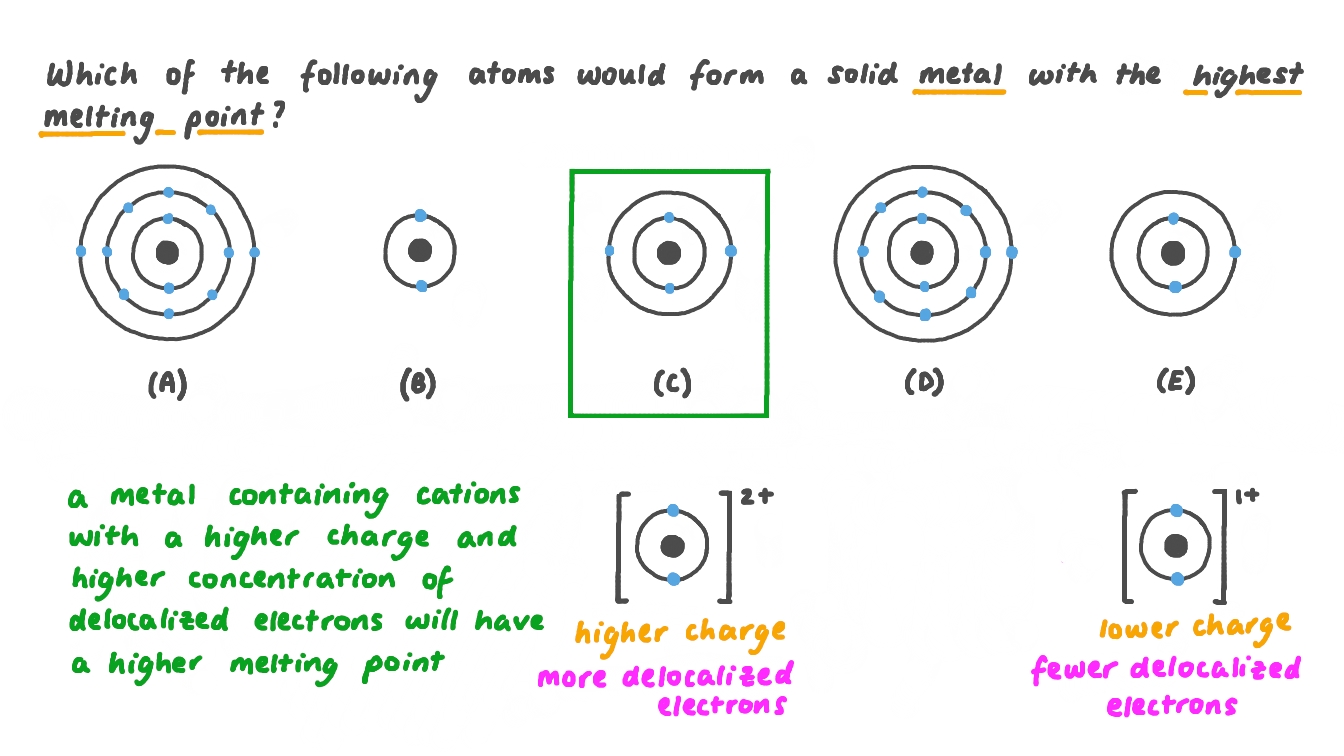
Question Video Identifying The System With The Highest Melting Point Nagwa

Additional Practice For Ch 10 And 11 Ophs Ap

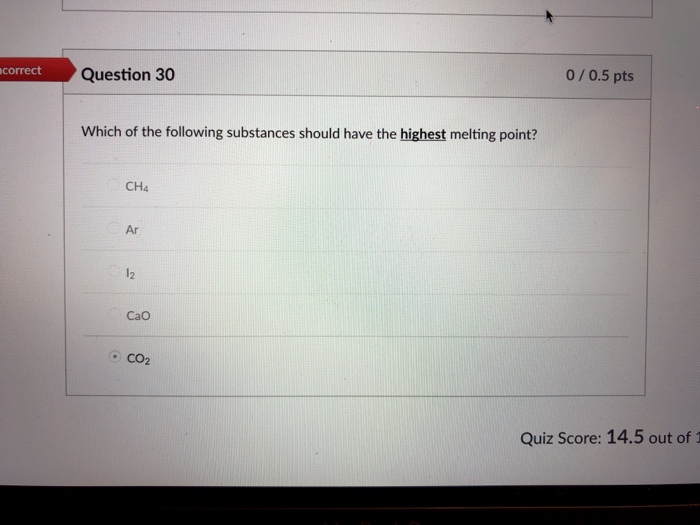
Solved Flag This Question Question 30 0 5 Pts Which Of The Chegg Com

Which Compound Has The Highest Melting Point Brainly Com
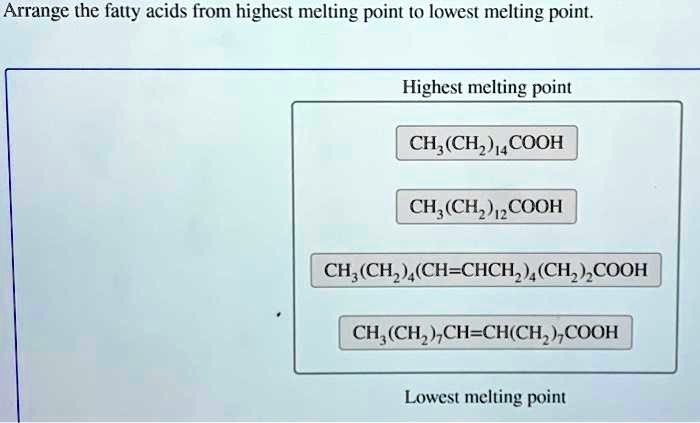
Solved Arrange The Fatty Acids From Highest Melting Point T0 Lowest Melting Point Highest Melting Point Ch Ch Cooh Ch Chz Jzcooh Ch Ch Ch Chch Ch Cooh Ch C Ch Ch Ch Ch Cooh Lowest Melting Point

Welcome To Learnapchemistry Com Ionic Bonding Ap Chem Ap Chemistry
Solved Correct Question 30 0 0 5 Pts Which Of The Following Chegg Com

Boiling Melting Points And Intermolecular Forces Youtube

Which Of The Following Substance Has The Highest Melting Point Youtube

Which Element Has The Highest Melting Point Seniorcare2share

Which Of The Following Substance Has The Highest Melting Point

Which Of The Following Has Highest Melting Point
How To Tell Which Substance Has The Highest Boiling Point Or Freezing Point Quora
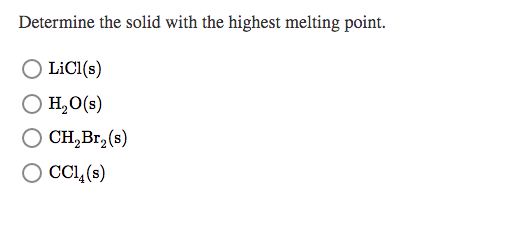
Solved Determine The Solid With The Highest Melting Point Chegg Com

Which Substance Has The Highest Lowest Melting Boiling Point Etc

Solved Question 2 Of 15 Which Of The Following Substances Chegg Com

Which Element Has The Highest Melting Point Seniorcare2share

Solved Which Of The Following Substances Should Have The Chegg Com
Comments
Post a Comment